Salesforce SAP Integration
Challenges of Pharmaceutical companies – how the combination of Salesforce plus SAP can help
Customer Relationship Management aka CRM
The start of Salesforce was CRM, but as we all know, today it is a lot more. CRM systems play a significant role in every industry and pharma is no exception. In this blog post we want to discuss common challenges and how Salesforce (as the system of engagement) and SAP (as the system of record) can help to solve them.
Clinical Trial Management
Before any new drug can be released to the market, clinical trials need to be performed. Salesforce offers Health Cloud to track the trial progress, participants, and regulatory documentation. In it, the relationship with trial patients, clinicians, and investigators. Should issues occur, they can be recorded in either Salesforce as a case or in SAP as a quality notification. Vigience Overcast provides this integration out-of-the-box.
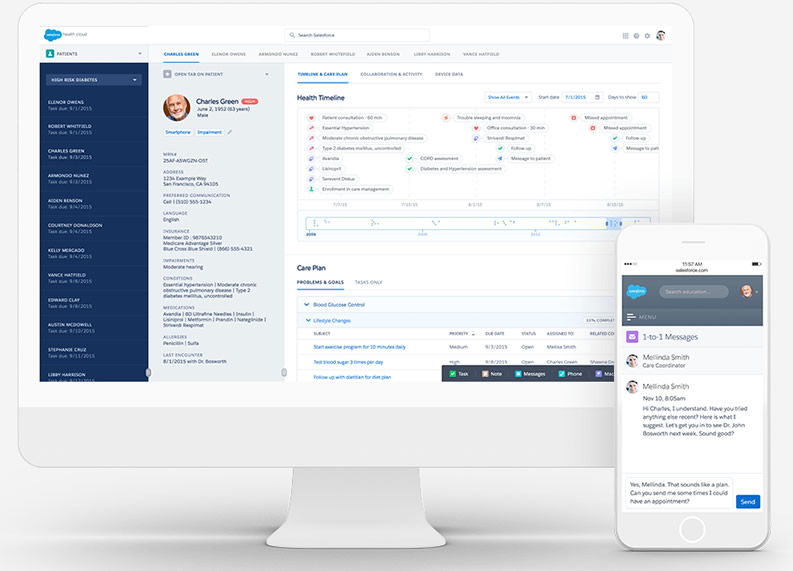
Regulatory Compliance
Nobody wants to take medicine that was not developed and produced under strict rules. Therefore, Pharmaceutical companies operate under strict regulatory frameworks (e.g., FDA, EMA), and Salesforce can support compliance efforts by:
- Automating document management workflows to ensure that all necessary documents, approvals, and audit trails are captured.
- Enforcing rules around sales rep interactions with healthcare providers to comply with anti-kickback laws and ethical guidelines (e.g., Sunshine Act reporting). Such financial benefits for providers can be recorded in SAP FI. It is essential to have this information visible in Salesforce so that no overspending happens.
- Integrating with systems like SAP ECC and S/4HANA can help to streamline compliance-related reporting, such as product recalls, or clinical trial data.
Batch Release Process
If a pharma company is releasing a batch, it must ensure that the quality is guaranteed. The person responsible for the release (Qualified Person) is personally liable for it.
Usually, the information about the batch is stored in many different systems – SAP is only one of them. This includes:
- Manufacturing must follow a pre-approved Master Batch Record (MBR), which outlines the exact steps, raw materials, and conditions for production.
- Once manufacturing is finished, Batch Manufacturing Records (BMR) are generated to document the actual production details, including any deviations or issues encountered (both 1 and 2 are recorded in SAP).
- In-Process and Final Product Testing happen during and after production. Any deviations must be documented, for example as an SAP QM notification.
- Document Review – the results from step 1 to 3 are reviewed, together with all associated documentation, including raw material certificates, environmental monitoring results, equipment cleaning records, and personnel training logs, is reviewed to ensure compliance with Good Manufacturing Practices (GMP).
- Deviation Reports are created that document any deviations from standard.
- A quality assurance (QA) review from a QA-team is performed and crucial to the release process.
- Regulatory compliance is checked and documented.
- The Batch Certification is performed by the Qualified Person (QP)

This process involves a lot more systems than just SAP. Here the Vigience Overcast support for MuleSoft comes in handy. Via MuleSoft, Overcast can access any backend system and collect all data to display it in the Salesforce UI.
Field Sales
Sales reps of pharma companies are often on the road to meet with healthcare providers (HCP) like clinics and pharmacies, therefore it is important for them to take their CRM with them on the road.
These reps can leverage the Salesforce Mobile app so that they have always access to critical customer information. As a lot of this information like invoice history and delivery status is stored in SAP, Vigience Overcast can be used to access in real-time from Salesforce in the browser, but also from the mobile app.
The Salesforce Mobile app
Track and Trace
Every package of medicine needs to be trackable by a unique serial number, often added as a QR code. This ensures that no counterfeit packages are sold and consumed. While the serial numbers are managed in SAP, the Salesforce platform and its community solution is allowing patients to access information on the drugs. Via an integration into SAP, we can allow patients but also HCPs to verify serial numbers and to report counterfeit products.
B2B Commerce
Hospitals, doctors and pharmacies can place orders, download invoices and check the delivery status via the Salesforce B2B Commerce Cloud. This leads to a better customer experience and to a reduced workload of the back-office team.
As many of these documents are stored in SAP, Overcast offers a B2B Commerce integration with an SAP-integrated shopping chart and check out process.
Support
Support via email, phone and other channels is very important for HCPs, but also for the patients. The Salesforce Service Cloud offers all the tools to ensure that the support team can work efficiently. New solutions like Agentforce and Einstein Copilot make it easier than ever to implement the support process and to reduce the workload of the support team.
Thanks to the Vigience Overcast integration into Agentforce, Einstein Copilot and Prompt Builder, the support team can now also access all information from SAP with natural language.
Conclusion
Salesforce and its Health Cloud is the right system of engagement for pharmaceutical companies that run SAP. With the help of Overcast, all information from SAP can easily be made available within the Salesforce user interface, either as Lightning UI components or as Actions in Einstein Copilot.
Get in touch with us if you want to learn how Vigience can help your organization to be more efficient by combining the power of Salesforce with SAP.










